Clinical Evidence
Results across our clinical development program demonstrate the safety and impact of Targeted Lung Denervation (TLD) using the dNerva® Lung Denervation System. Detailed information regarding Nuvaira clinical studies can also be found at www.clinicaltrials.gov.
dNerva® Clinical Development Program
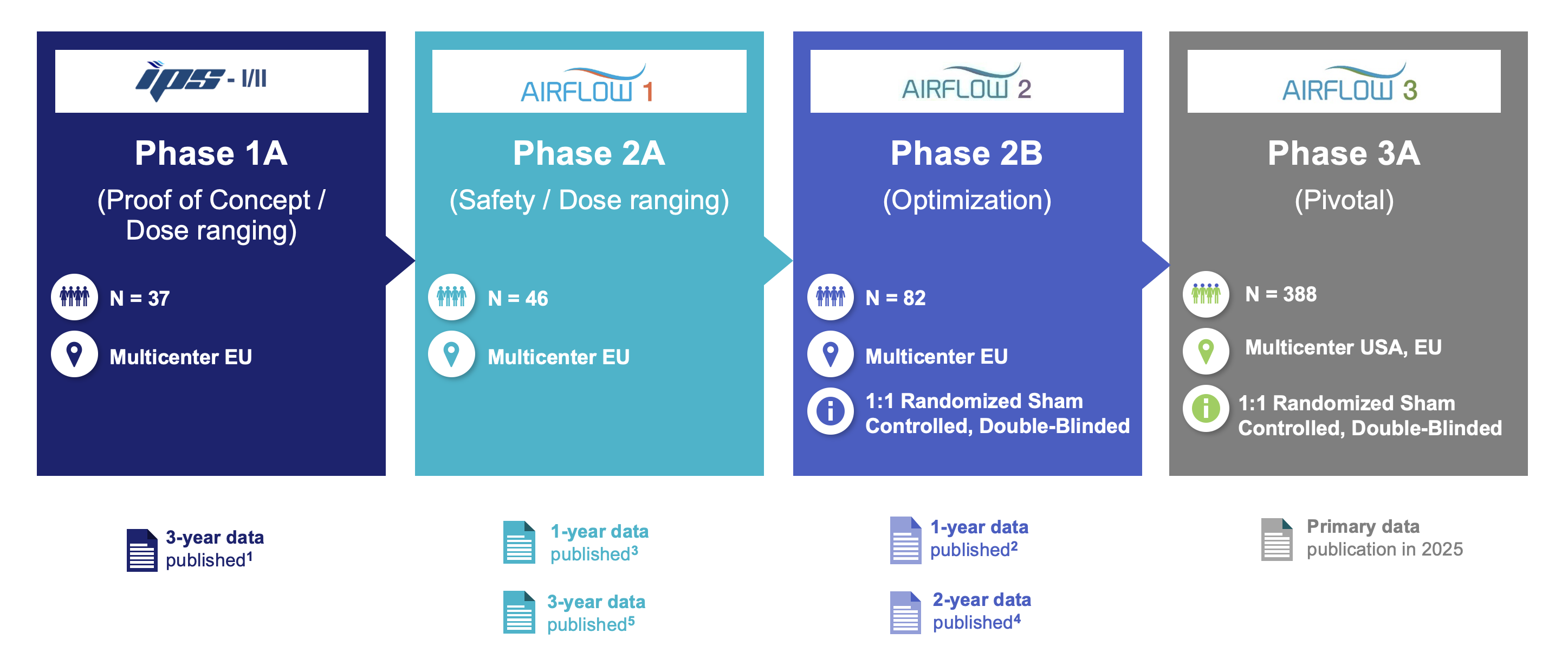
Studies
Over 500 patients have been treated in Nuvaira’s clinical studies.
Find out more about each of the studies in Nuvaira’s clinical development program.
Published in the International Journal of Chronic Obstructive Pulmonary Disease, November 20202
The clinical impact of TLD is durable, as it is found to reduce the risk of serious COPD adverse events over 2 years with stabilized lung function. The reduction of severe COPD exacerbations has the potential to improve long-term clinical outcomes and reduce healthcare costs.
AIRFLOW-2 Study Conclusions
Robust study design
Positive Safety Profile
TLD demonstrated a significantly lower risk of hospitalized COPD exacerbations
Lung function and quality of life remained stable over 2 years
Published in Respiratory Research, February 202121
TLD in COPD patients demonstrated a positive safety profile out to 3 years, with no late-onset serious adverse events related to denervation therapy. Clinical stability in lung function, quality of life, and exacerbations were observed in TLD treated patients over 3 years of follow up.



